Dr. Ayyavoo is a molecular virologist with over three decades of expertise in HIV-1 virology. Her research laboratory is dedicated to unraveling the intricate mechanisms through which HIV-1 instigates pathogenesis in humans, impacting both the peripheral and central nervous systems (CNS) of people living with HIV (PWH). Leveraging autopsied tissue samples, her laboratory has conducted pioneering investigations revealing substantial alterations in neurons among HIV patients experiencing cognitive disorders, known as HIV-associated neurocognitive disorder (HAND), as opposed to HIV patients without such complications. Her research interests are:
Developing brain organoid model to study HIV-1 associated neurocognitive disorder: Understanding and combating HIV-associated 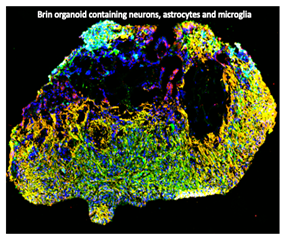 neurocognitive disorder (HAND) is of paramount importance given its increasing prevalence among people living with HIV (PLWH) who are under therapy. However, studying the cellular and molecular events underlying HIV neuropathogenesis poses significant challenges due to the intricate neural-glial networks that operate in tandem within the brain. Despite these challenges, ongoing research endeavors are aimed at unraveling the complexities of CNS diseases, including HAND, through innovative approaches. One such approach involves the utilization of advanced in vitro models, such as brain organoids, which offer a platform for studying neurodegenerative diseases in a controlled laboratory environment. While replicating the intricate neural-glial interactions of the brain in vitro remains a daunting task, brain organoids provide a promising avenue for investigating the cellular and molecular mechanisms involved in HIV neuropathogenesis. Brain organoids, sophisticated three-dimensional miniature models of the human brain grown from stemcells, represent a promising tool for studying neurodegenerative diseases. These organoids offer a unique platform that closely mimics the cellular architecture and functionality of the human brain, providing researchers with the opportunity to observe the progression of neurodegenerative diseases in a controlled laboratory setting. Overall, brain organoids serve as a powerful and ethically viable model system that holds great promise for advancing our understanding and treatment of neurodegenerative diseases. Dr. Ayyavoo’s laboratory is the first to develop the brain organoids with microglia.
neurocognitive disorder (HAND) is of paramount importance given its increasing prevalence among people living with HIV (PLWH) who are under therapy. However, studying the cellular and molecular events underlying HIV neuropathogenesis poses significant challenges due to the intricate neural-glial networks that operate in tandem within the brain. Despite these challenges, ongoing research endeavors are aimed at unraveling the complexities of CNS diseases, including HAND, through innovative approaches. One such approach involves the utilization of advanced in vitro models, such as brain organoids, which offer a platform for studying neurodegenerative diseases in a controlled laboratory environment. While replicating the intricate neural-glial interactions of the brain in vitro remains a daunting task, brain organoids provide a promising avenue for investigating the cellular and molecular mechanisms involved in HIV neuropathogenesis. Brain organoids, sophisticated three-dimensional miniature models of the human brain grown from stemcells, represent a promising tool for studying neurodegenerative diseases. These organoids offer a unique platform that closely mimics the cellular architecture and functionality of the human brain, providing researchers with the opportunity to observe the progression of neurodegenerative diseases in a controlled laboratory setting. Overall, brain organoids serve as a powerful and ethically viable model system that holds great promise for advancing our understanding and treatment of neurodegenerative diseases. Dr. Ayyavoo’s laboratory is the first to develop the brain organoids with microglia.
Understanding HIV latency in CNS and to develop therapeutic targets to activate the virus: Despite significant progress in managing HIV-1 infection, eradicating the virus entirely remains challenging. Infected cells persist in various tissue compartments, including the brain, even during combination antiretroviral therapy (ART). The central nervous system (CNS) serves as a viral reservoir, with cellular compartmentalization and limited penetrability of antiretroviral drugs due to the blood-brain barrier. Primary reservoir cells in the CNS include microglia, perivascular macrophages, and astrocytes, yet our understanding of HIV-1 infection in CNS reservoirs remains limited. Studying CNS latency is hindered by the lack of suitable HIV-CNS models. Utilizing a 3D brain organoid model with human microglia sourced from HIV-1 positive donors, we aim to characterize transcriptional changes. This knowledge may lead to the discovery of novel therapeutic interventions targeting latency-inducing and/or silencing antivirals for the CNS compartment. Additionally, we aim to validate and assess whether these target molecules can traverse the blood-brain barrier and effectively reach their intended cellular targets in the brain.
1991 | Madurai Kamaraj University, India | Doctor of Philosophy
1991-1993 | The Wistar Institute | Postdoctoral Fellow
1994-1998 | The University of Pennsylvania | Research Associate
IDM 2001 - Molecular Biology of Microbiol Pathogens
IDM 2023 - Laboratory Methods
IDM 2025 - Seminar Series & Journal Club
IDM 2010 - Pathogen Biology
IDM 2014 - Functional Genomics
- Dos Reis RS, Selvam S, Wagner MCE, Ayyavoo V. (2024). Modeling HIV-1 infection in CNS via infected monocytes using immunocompetent 3D-brain organoids. Methods Mol Biol. (in press).
- Dos Reis RS, Sant S, Ayyavoo V. (2023). Three-dimensional human Brain Organoids to model HIV-1 Neuropathogenesis. Methods Mol Biol. 2610:167-178.
- Ayyavoo, V. (2021). Modeling Human Viral Diseases: Trials and Triumphs. Frontiers in Virology, Editorial comments in launching the journal.
- Dos Reis RS, Sant S, Keeney H, Wagner MCE, Ayyavoo V. (2020). Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia. Scientific Reports 10:15209.
- Guha D, Wagner MCE, Ayyavoo V. (2018). Human immunodeficiency virus type 1 (HIV-1)-mediated neuroinflammation dysregulates neurogranin and induces synaptodendritic injury. J. Neuroinflammation. 15:126.
- Venkatachari NJ, Jain S, Walker L, Bivalkar-Mehla S, Chattopadhyay A, Bar-Joseph Z, Rinaldo C, Ragin A, Seaberg E, Levine A, Becker J, Martin E, Sacktor N, Ayyavoo V. (2017). Transcriptome analyses identify key cellular factors associated with HIV-1 associated neuropathogenesis in infected men. AIDS. 31: 623.
- Guha D, Mancini A, Sparks J, Ayyavoo V. (2016). Human Immunodeficiency Virus type 1 (HIV-1) infection dysregulates expression of cell cycle protein p21 in CD4+ T cells through miR-20a and miR-106b. J Cell. Biochem. 117:1902.
- Venkatachari NJ, Jain S, Mancini AE, Chattopadhyay A, Bar-Joseph Z, Ayyavoo V. (2015). Temporal transcriptional response to Latency reversing agents identifies specific factors regulating HIV-1 viral transcriptional switch: Cell line-based model. Retrovirology. 12:85.
For a full list publications, see PubMed
